Duoflora – Bifidobacterium Breve M16V
Bifidobacterium Breve M16V is a specific probiotic that stands out for its extensive benefits and potential to support overall well-being. This probiotic strain, derived from the healthy gut flora of infants, has been extensively studied and is recognized for its unique properties. This write up summarizes the features and benefits of Bifidobacterium Breve M16V to demonstrate why it’s considered a probiotic powerhouse.
Features of Bifidobacterium Breve M16V
Bifidobacterium Breve M16V is a human strain probiotic isolated from the intestines of healthy, breastfed infants, ensuring it is a naturally occurring and safe probiotic strain. It is classified as Generally Recognized as Safe (GRAS) by regulatory authorities, underlining its safety for consumption.
Stability and Viability:
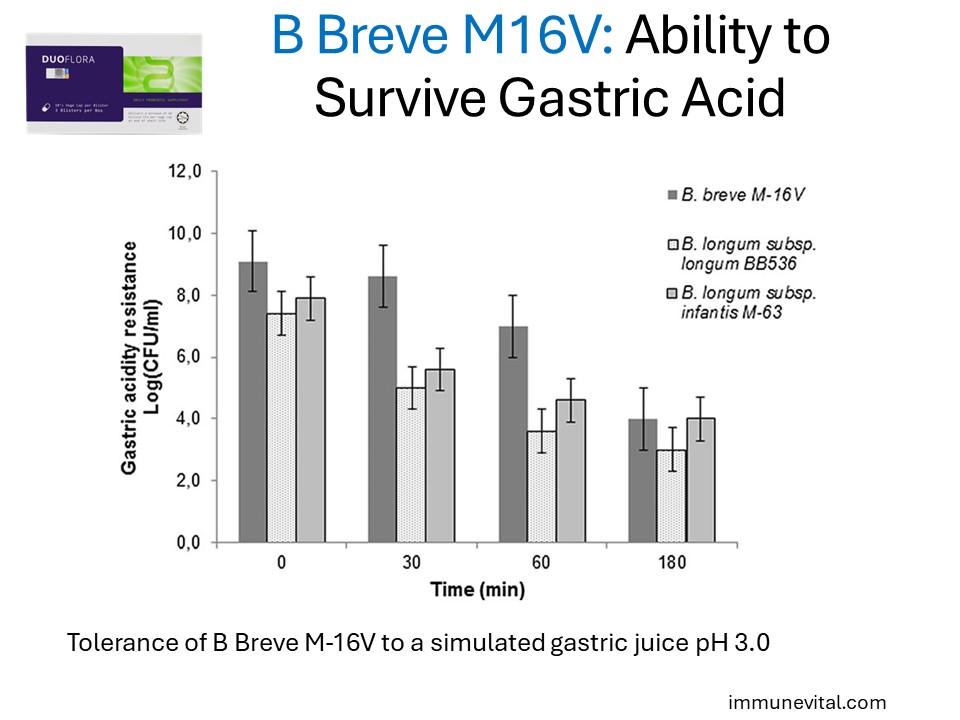
This strain is known for its stability and ability to survive the acidic environment of the stomach ensuring it reaches the intestines alive.
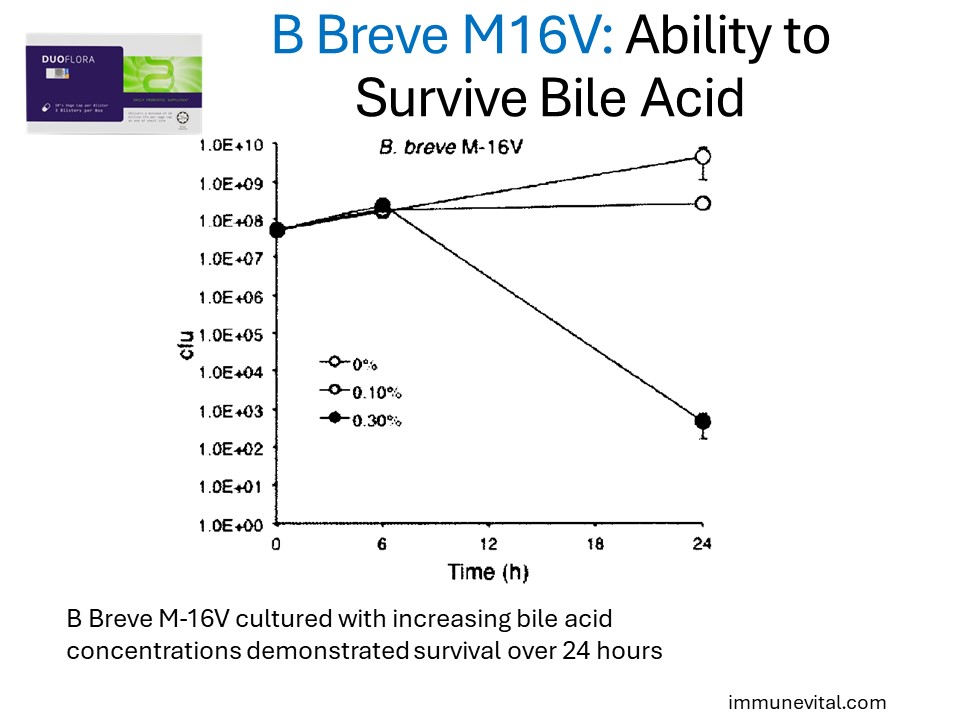
This resilience also extends towards bile acids found in the small intestine.
These survival factors are key to its efficacy in promoting gut and immune health.
Why it is important to choose a Human strain Probiotic that is compatible with Human Milk
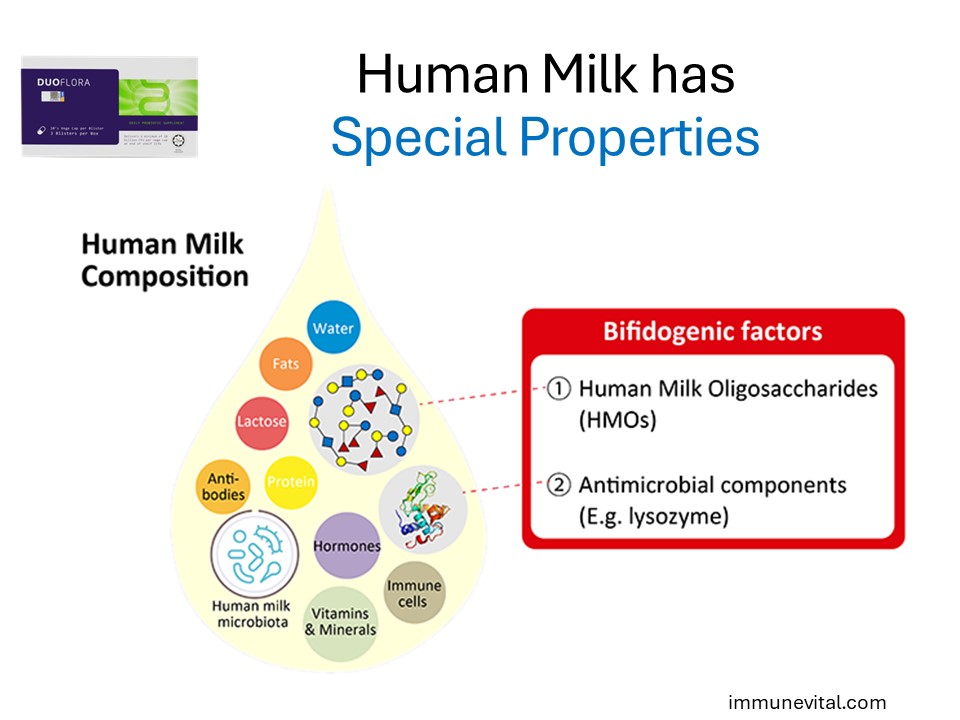
Human milk is the gold standard in infant nutrition. It plays an enormous role in infant health development. Its secret is its ingenious and unique composition. Human milk is comprised of vital nutrients and distinct bioactive molecules that the infant needs for growth and development. Take note of the antimicrobial components that make a significant portion of breast milk to protect the infant.
Duoflora: Bifidobacterium Breve M16V is compatible with Human Milk
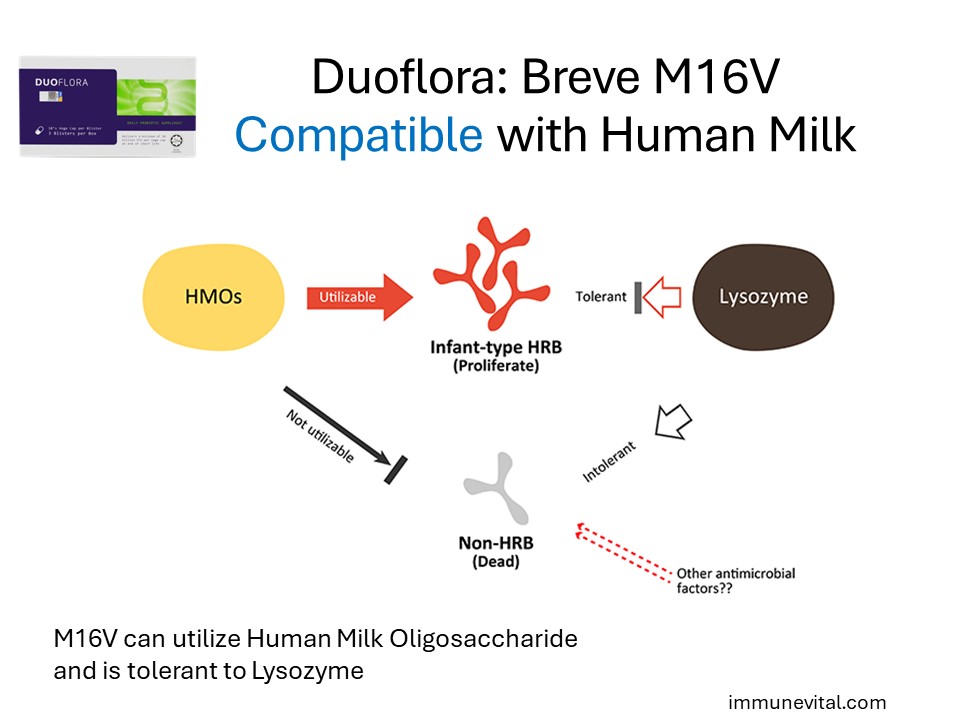
Lysozyme in Human Breast Milk kills bacteria except Human Residential Bifidobacteria (HRB). B Breve M16V in Duoflora can utilize a prebiotic in human milk called HMO and is tolerant to lysozyme.
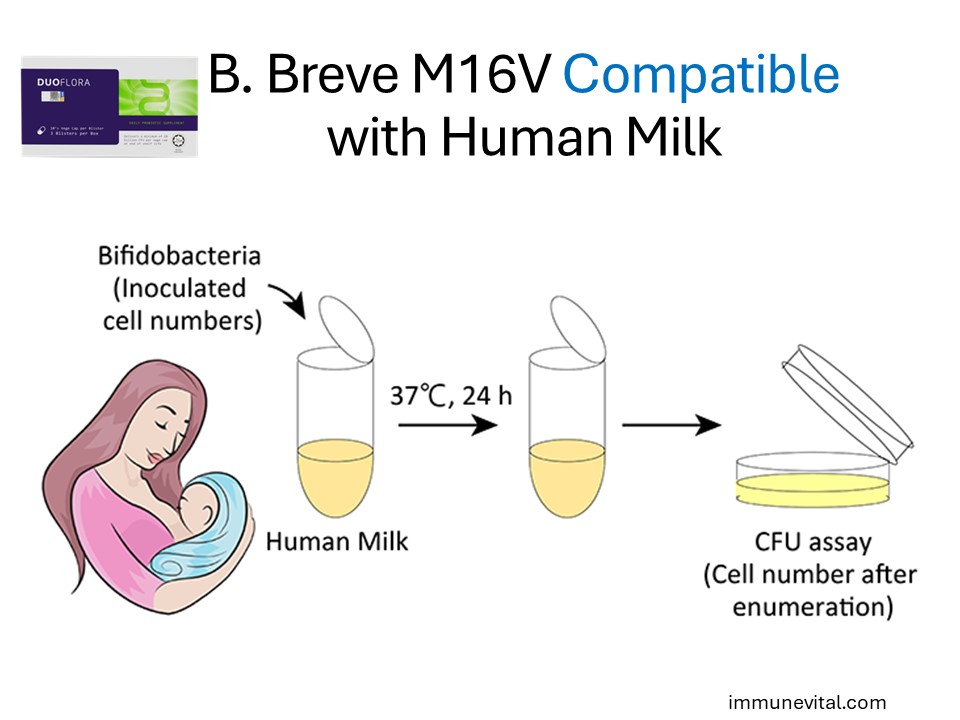
In a study by Minami, human breast milk is selective of probiotic strains that are beneficial for infant health. One of the key strains is Bifidobacterium Breve M16V. In this study, non-human derived probiotic strains failed to grow and even died out.
Choose a HUMAN strain Probiotic for better Health Benefits
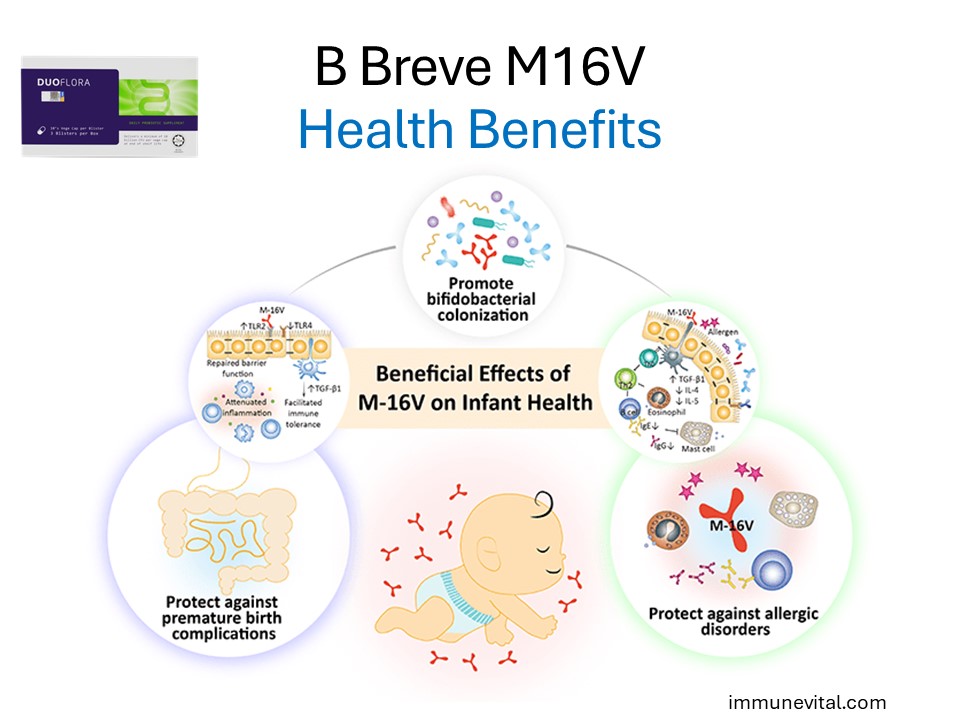
Benefits of Bifidobacterium Breve M16V
Gut Health and Digestion
Bifidobacterium Breve M16V plays a crucial role in maintaining a balanced gut microbiome. It aids in the digestion of dietary fibers and complex carbohydrates, promoting regular bowel movements and preventing digestive discomfort.
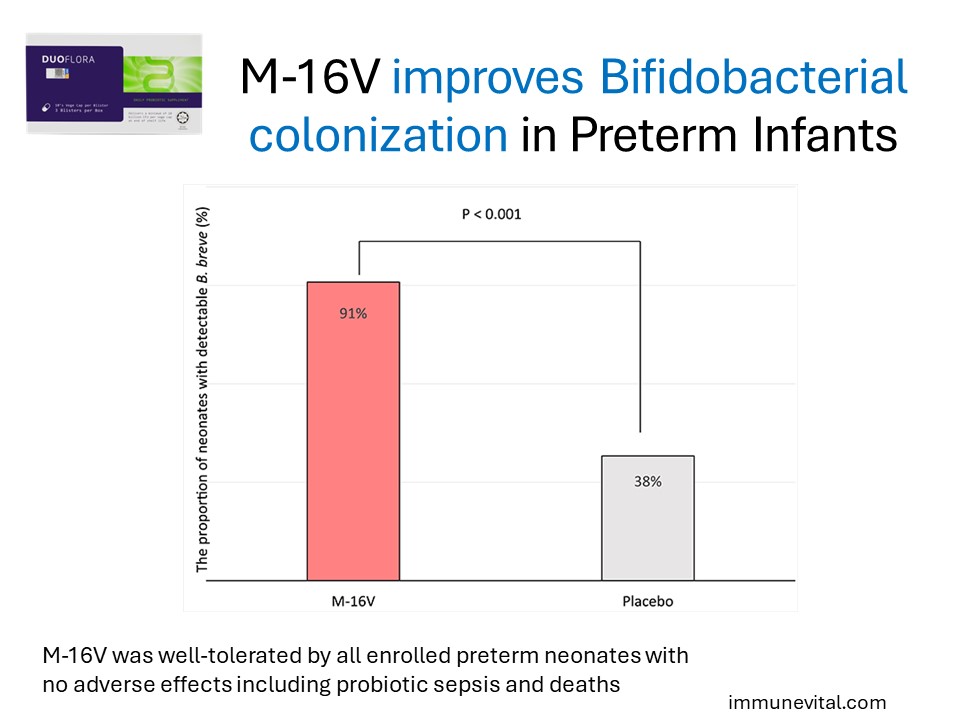
The gut microbiome plays a crucial role in protecting infants. Preterm infants are especially vulnerable as the gut barrier is not yet established. Breve M16B helps establish a healthy Bifidobacterial colonization that is safe for the infant.
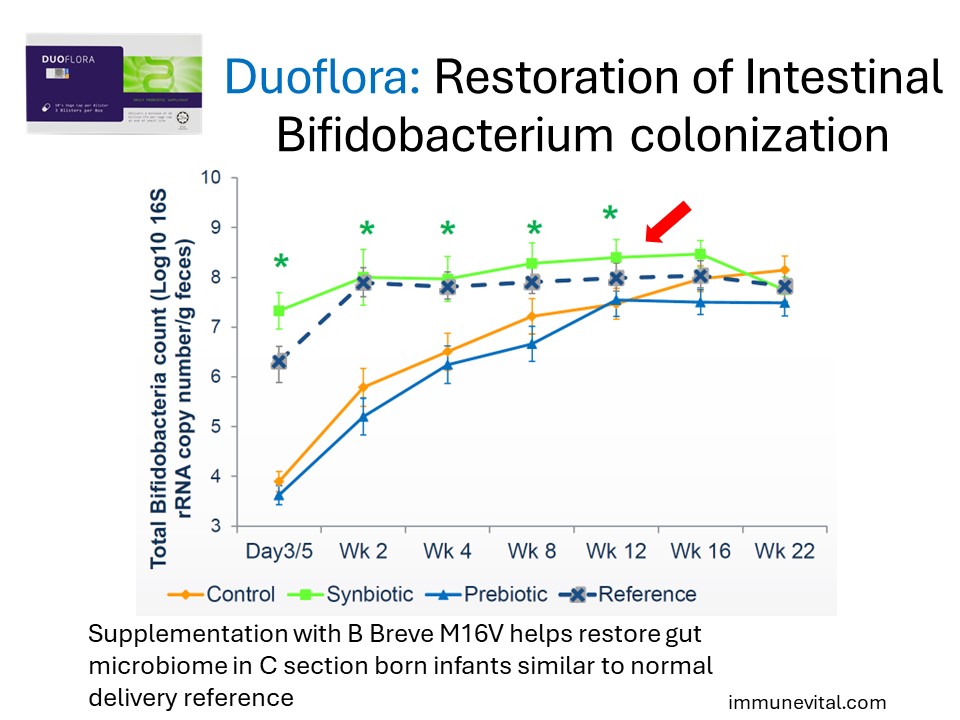
Infants born via Caesarean Section are found to have abnormal gut microbiome which can lead to allergies and delayed immune development. Supplementation with Breve M16V has been shown to help.
Protecting Intestinal Barrier Function
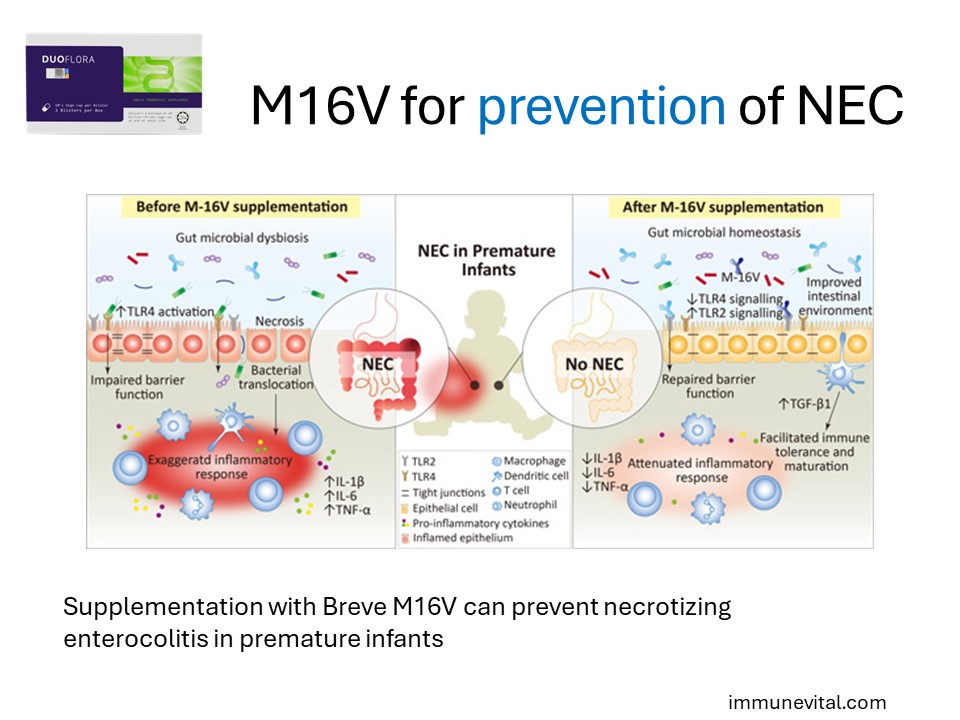
Premature infants are at elevated risk to develop multiple health comorbidities; one of which is the devastating necrotizing enterocolitis (NEC). Although the exact etiology and pathogenesis of NEC remain elusive, perturbation of the gut microbiota and a lack of bifidobacteria, leading to a hyperinflammatory response, appears to be a key factor that predisposes neonates to NEC
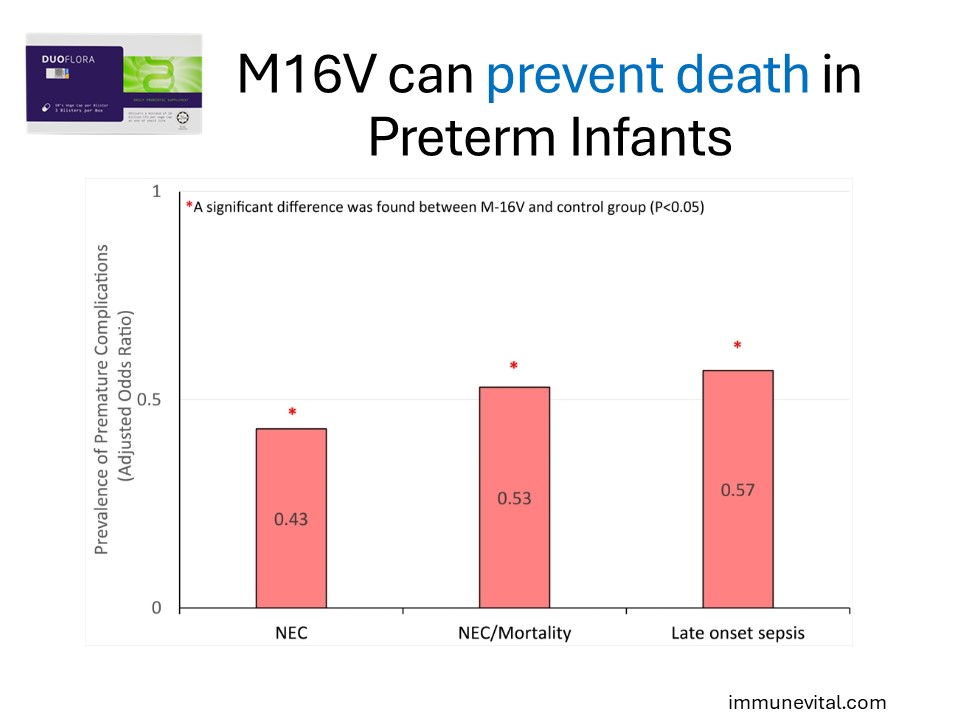
In a retrospective cohort study involving 835 preterm neonates as normal control and 920 preterm neonates receiving M-16V supplementation with enteral feeds until the corrected age of 37 weeks, M-16V significantly lowered the incidence of “NEC Stage II”, “NEC ≥ Stage II or all-cause mortality”, late onset sepsis and age at full feeds. These clinical findings underscore the potential roles of M-16V as a promising infant probiotic that could potentially impact the incidence, morbidity and mortality associated with NEC.
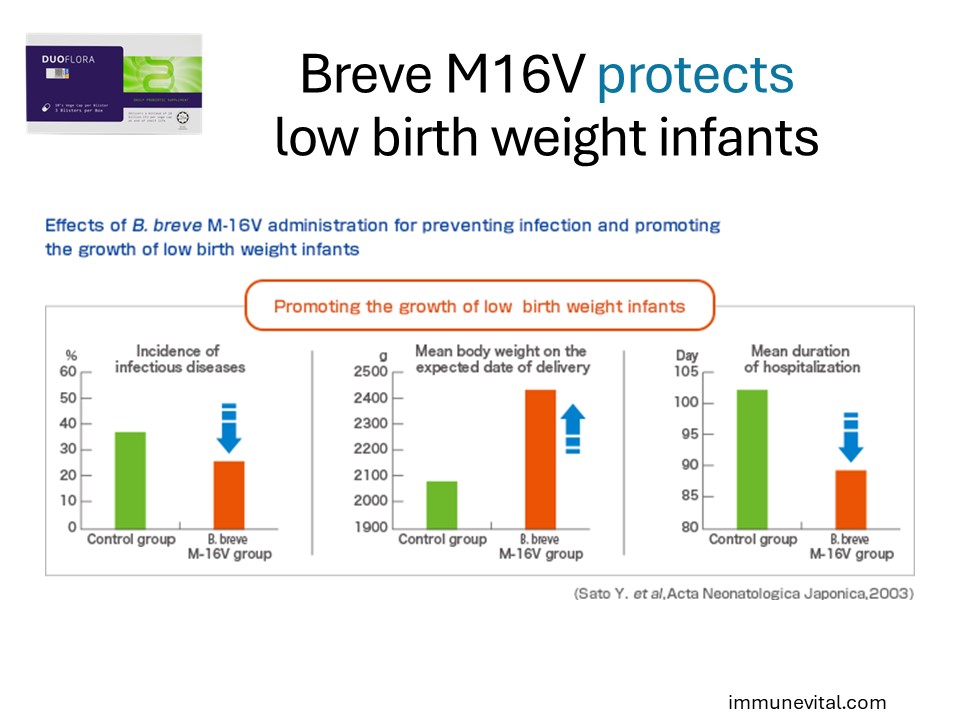
Bifidobacterium Breve M16V is particularly beneficial for preterm and low birth weight infants. It aids in preventing infections and supports healthy weight gain and overall development.
In the study above, One hundred and sixty-two low birth weight infants, weighing less than 1,500 g, were divided into two groups: one group was administered B. breve M-16V and the other (control) was not. As a result, the incidence of infectious diseases was inhibited by B. breve M-16V administration. Furthermore, the body weight on the expected date of delivery was increased by B. breve M-16V administration, resulting in a shorter duration of hospitalization.
Modulates Allergic Responses
Allergy Alleviation: B Breve M16V can help in reducing the severity of allergic reactions. It works by modulating immune responses and decreasing the production of pro-inflammatory cytokines that contribute to allergic symptoms.
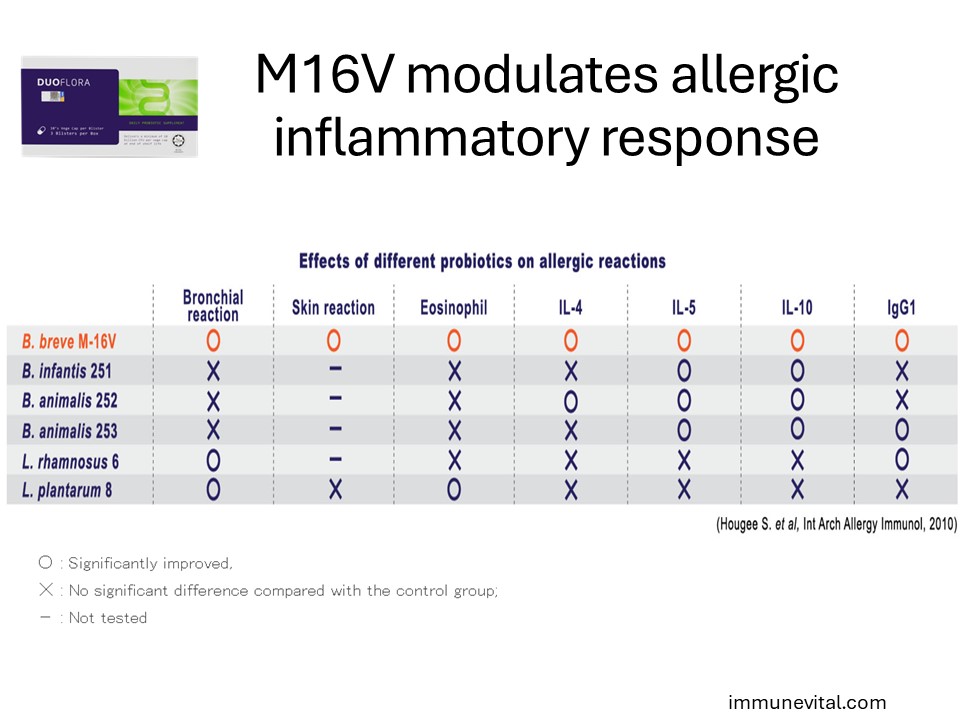
In the study above, the anti-allergy activity of Breve M-16V was compared with that of other bifidobacterial strains and lactic acid bacteria. Breve M-16V was found to be the most potent for anti-allergy.
Helps in Eczema
Eczema Relief: Research has shown that Bifidobacterium Breve M16V can be effective in managing symptoms of eczema. It reduces skin inflammation and supports the skin’s barrier function, leading to fewer flare-ups and improved skin health.
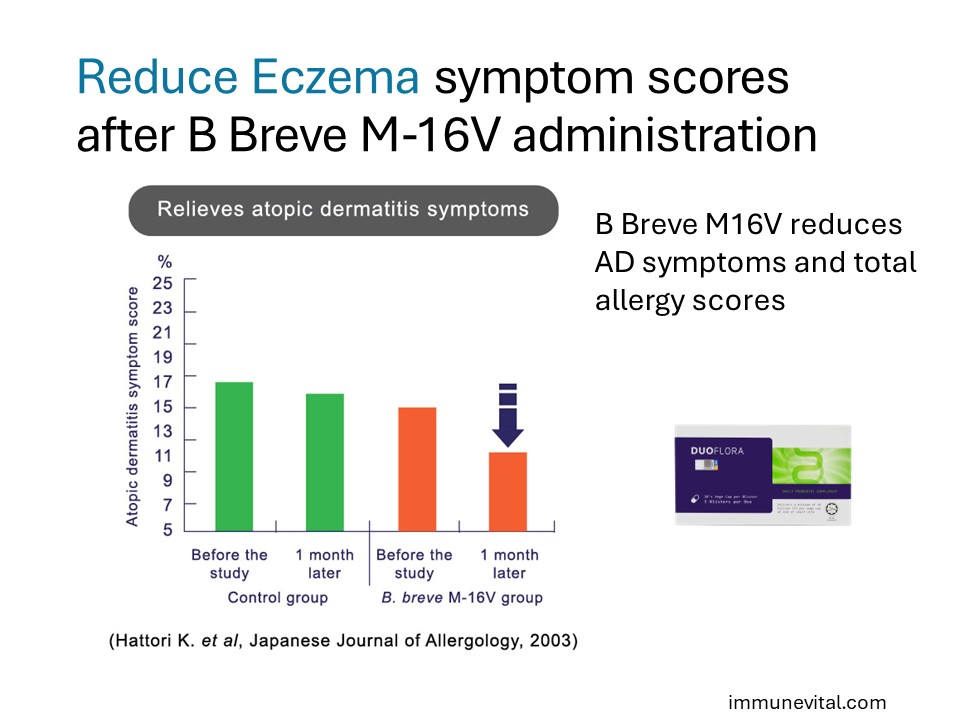
A randomized controlled trial above investigated B. Breve M16V in 15 infants with eczema. They were split to receive either a probiotic or placebo and took their respective supplement for one month. In the probiotic group, researchers found significant improvements in the proportion of Bifidobacteria and allergic symptoms (cutaneous symptom score and total allergic score). This study demonstrates that Breve M16V helps improve eczema in infants.
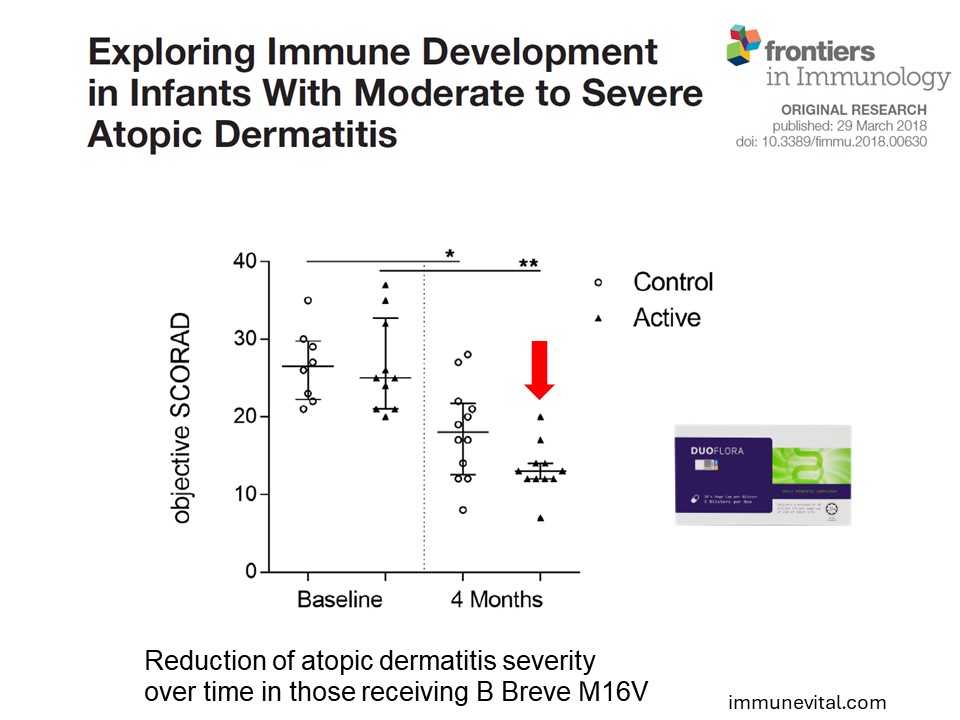
In a gold standard trial above, 31 infants under the age of 11 months with severe atopic dermatitis were randomized to receive Breve M16V daily or placebo for 4 months. At the end of the study, there was a significant eczema improvement in the probiotic group (p<0.01). There was also a significant reduction in the Th2 to Th1 chemokine ratio (p<0.01) in the probiotic group compared with placebo, indicating a reduction in Th2 immune response. This study demonstrates the ability for Breve M16V to help in infants with moderate and severe eczema.
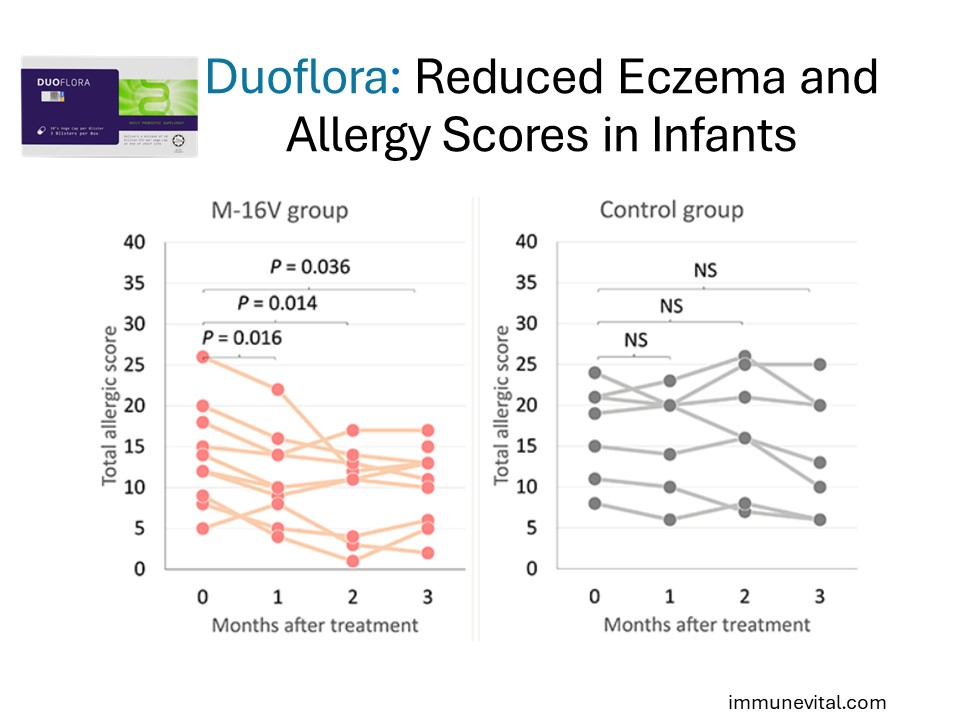
In the study above, Breve M16V probiotic was given to infants with cow milk allergy and eczema. It demonstrated the ability of Breve M16V to improve eczema and total allergic scores in infants with eczema and concurrent food allergy.
Helps in Respiratory Allergies
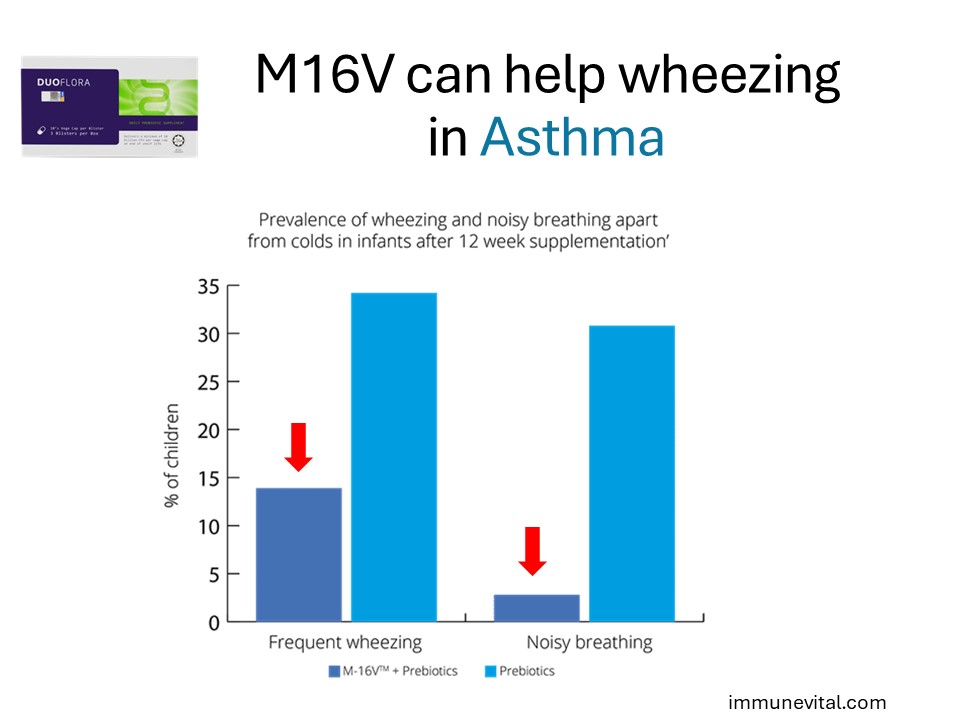
Above was a double-blind, randomized placebo-controlled study investigated Breve M16V in 75 infants (over 7 months old) with eczema who were suffering with asthma like symptoms. The infants received probiotic supplement for 12 weeks and follow up analysis was conducted after one year. At follow up, the prevalence of ‘frequent wheezing’ was significantly lower in the Breve M16V probiotic group than the control group (13.9% v 34.2% respectively). Wheezing and/or noisy breathing apart from colds had significantly reduced in the probiotic group compared with control (2.8% v 30.8% respectively). This suggests that Breve M16V can improve the immune system and reduce asthmatic wheezing even 1 year after administration.
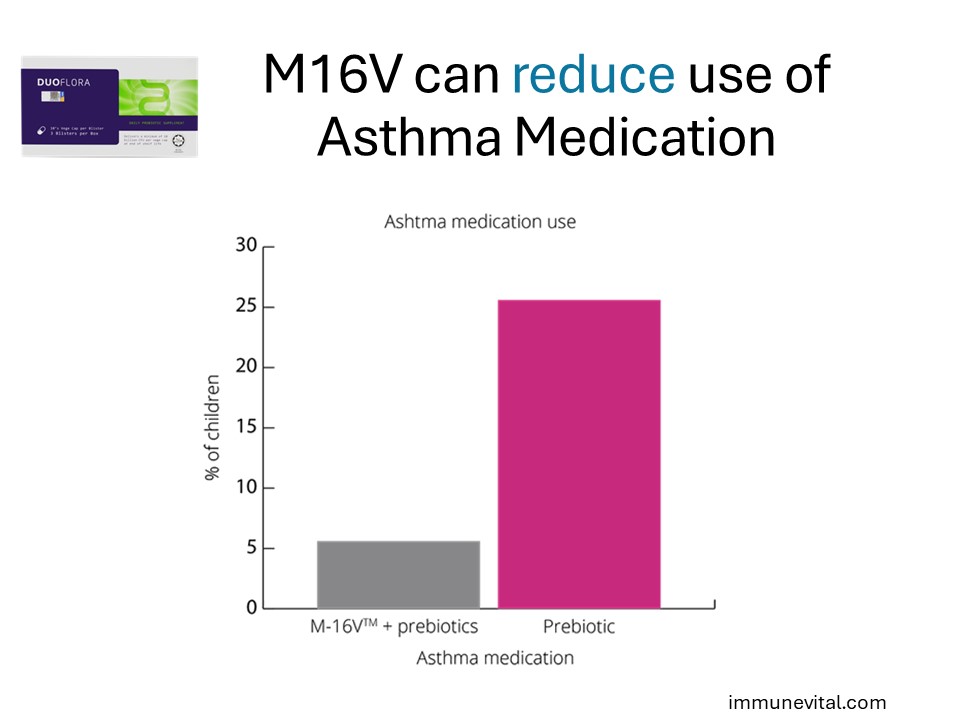
In the same study above, children that received probiotic were significantly less likely than those in the placebo group to use asthma medications. This suggests that Breve M16V may be able to prevent the development of asthma.
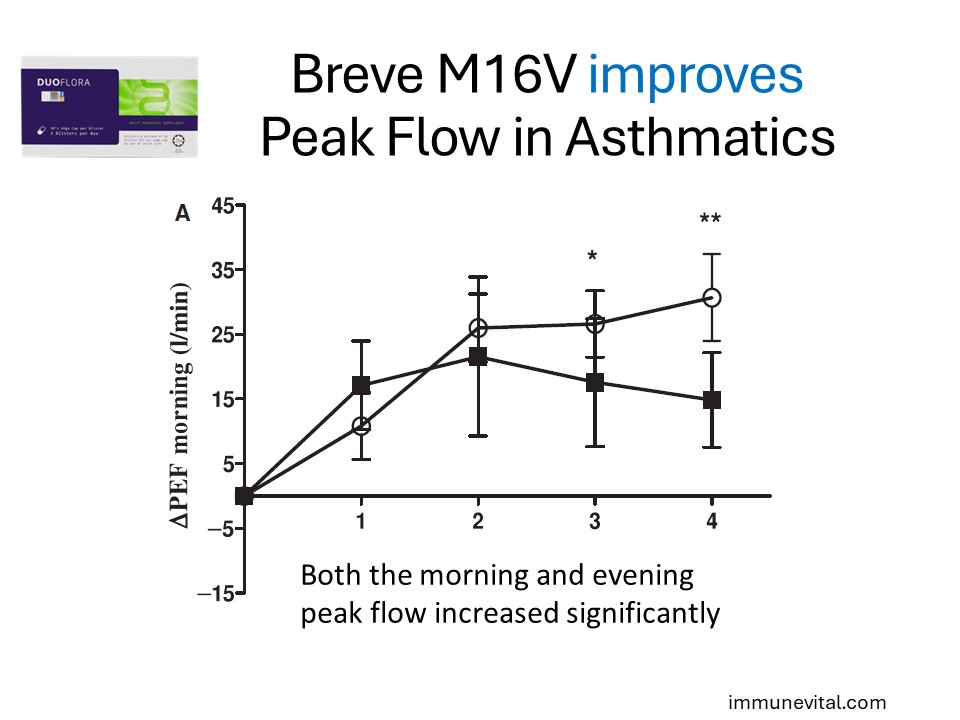
In the study above, twenty-nine patients with asthma and house dust mite allergy were randomized in a double-blind, parallel design to receive placebo or probiotics for 4 weeks. Four-week treatment with probiotics reduced systemic production of Th2-cytokines after allergen challenge and improved Peak Flows. This indicates that Breve M16V can help modulate the allergic inflammation of asthma and help improve lung function.
Prevention of Eczema
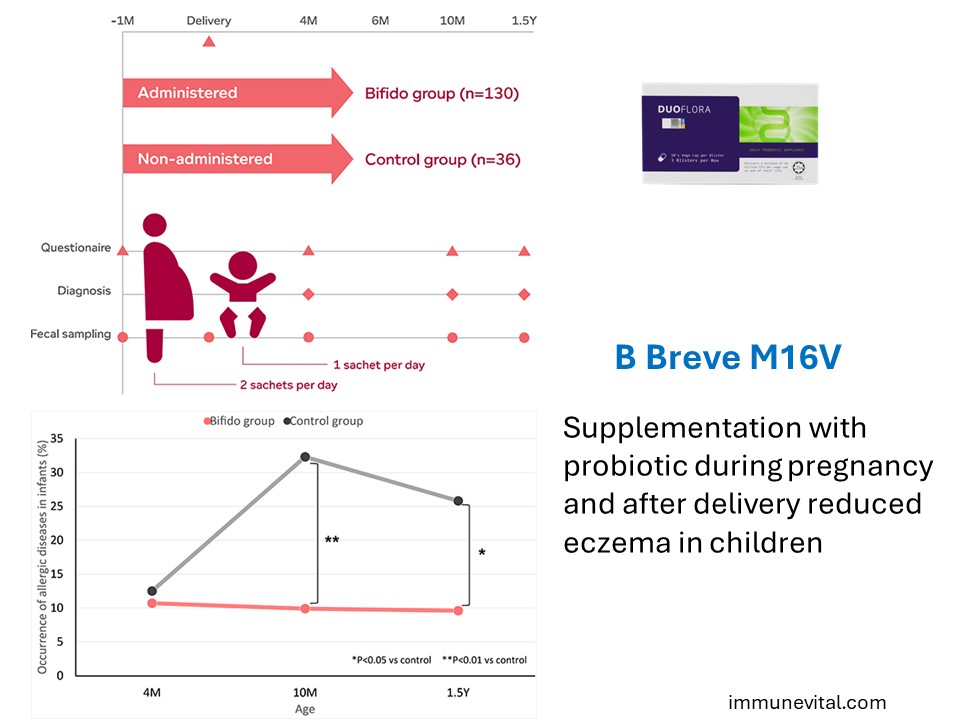
Prenatal and postnatal supplementation with Bifidobacteria probiotic mixture significantly reduced the risk of developing eczema in infants during the first 18 months of life as compared to the control group. These findings implicate that supplementation with Bifidobacterial probiotic mixture containing Breve M16V can modulate both maternal and neonatal gut microbiota for prevention of infant allergies.
Conclusion
Bifidobacterium Breve M16V is a versatile and potent probiotic strain with a wide range of health benefits:
- Human strain probiotic that is compatible with Human Milk
- Proven ability to survive gastric acid and bile acid to reach the gut alive to provide health benefits
- Helps infants delivered via Caesarean section to acquire a normal gut microbiome
- Protects the gut of preterm infants and low birth weight infants from necrotizing enterocolitis and improve survival rates
- Able to modulate allergic inflammatory responses
- Reduces wheezing in asthmatics and improve lung function
- Prevent asthma development in infants with wheezing
- Prevent eczema development in infants
Science for your Health
References:
Witkowska-Zimny M, Kaminska-El-Hassan E. Cells of human breast milk. Cell Mol Biol Lett. 2017;22:11. doi:10.1186/s11658-017-0042-4
Wong, C.B., Iwabuchi, N. and Xiao, J.Z., 2019. Exploring the Science behind Bifidobacterium breve M-16V in Infant Health. Nutrients, 11(8), p.1724.
Patole, S.K., Rao, S.C., Keil, A.D., Nathan, E.A., Doherty, D.A. and Simmer, K.N., 2016. Benefits of Bifidobacterium breve M-16V supplementation in preterm neonates-a retrospective cohort study. PloS one, 11(3), p.e0150775.
Minami, J., Odamaki, T., Hashikura, N., Abe, F. and Xiao, J., 2016. Lysozyme in breast milk is a selection factor for bifidobacterial colonisation in the infant intestine. Beneficial Microbes 7: 53-60.
Bergmann H, Rodríguez JM, Salminen S, Szajewska H. Probiotics in human milk and probiotic supplementation in infant nutrition: a workshop report. British Journal of Nutrition. 2014;112(7):1119-1128. doi:10.1017/S0007114514001949
Toscano, M., De Vecchi, E., Gabrieli, A. et al. Probiotic characteristics and in vitro compatibility of a combination of Bifidobacterium breve M-16 V, Bifidobacterium longum subsp. infantis M-63 and Bifidobacterium longum subsp. longum BB536. Ann Microbiol 65, 1079–1086 (2015).
Patole, S., Keil, A.D., Chang, A., Nathan, E., Doherty, D., Simmer, K., Esvaran, M. and Conway, P., 2014. Effect of Bifidobacterium breve M-16V supplementation on fecal bifidobacteria in preterm neonates-a randomised double blind placebo controlled trial. PloS one, 9(3), p.e89511.
Lay C, et al. A synbiotic intervention modulates meta-omics signatures of gut redox potential and acidity in elective caesarean born infants. BMC Microbiol. 2021 Jun 25;21(1):191.
Sato Y et al., 「Effects of administration of bifidobacteria in premature infants」Acta Neonatologica Japonica 39(2): 247 (2003)
Hougee S. et al, Oral treatment with probiotics reduces allergic symptoms in ovalbumin-sensitized mice: A bacterial strain comparative study. Int Arch Allergy Immunol 151: 107-117 (2010)
Hattori K et al., 「 Effects of administration of bifidobacteria on fecal microflora and clinical symptoms in infants with atopic dermatitis」Japanese Journal of Allergology52(1): 20-30 (2003)
Hulshof L, Overbeek SA, Wyllie AL, et al. Exploring immune development in infants with moderate to severe atopic dermatitis. Front Immunol. 2018;9
Taniuchi S, Hattori K, Yamamoto A, Sasai M, Hatano Y, Kojima T, et al. Administration of Bifidobacterium to infants with atopic dermatitis: Changes in fecal microflora and clinical symptoms. J Applied Res. 2005;5: 387-396.
Van der Aa LB, van Aalderen WM, Heymans HS, Henk Sillevis Smitt J, Nauta AJ, Knippels LM, Ben Amor K, Sprikkelman AB; Synbad Study Group. Synbiotics prevent asthma-like symptoms in infants with atopic dermatitis. Allergy. 2011 Feb;66(2):170-7
Van de Pol MA, Lutter R, Smids BS, Weersink EJM, van der Zee JS. Synbiotics reduce allergen-induced T-helper 2 response and improve peak expiratory flow in allergic asthmatics. Allergy 2011; 66: 39–47
Enomoto, T., Sowa, M., Nishimori, K., Shimazu, S., Yoshida, A., Yamada, K., … & Odamaki, T. (2014). Effects of bifidobacterial supplementation to pregnant women and infants in the prevention of allergy development in infants and on fecal microbiota. Allergology International, 63(4), 575-585.